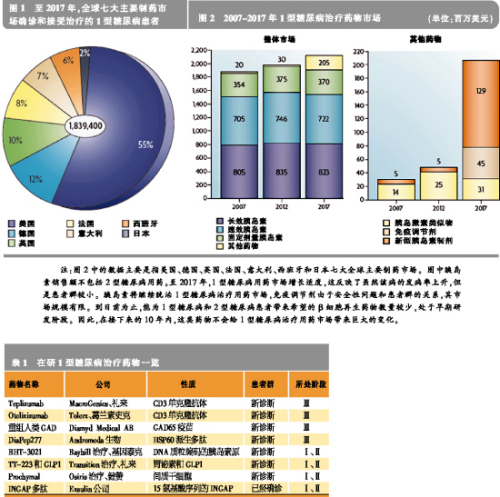
Type I diabetes (T1D), also known as juvenile or insulin-dependent diabetes, is an autoimmune and metabolic disease characterized by T cells that cause pancreatic beta cell damage, which in turn causes insulin secretion and hyperglycemia. In 2007, there were 437,500 children with type I diabetes worldwide. Each year, 70,000 children under 14 years of age with type 1 diabetes are newly added in the world. The annual growth rate is 3%, especially in younger children. At present, the etiology of type I diabetes is still not clear. It is generally believed that genetic genetic defects, environmental factors, and metabolic changes are the main causes of the disease's occurrence and development. The lack of insulin in Type I diabetes leads to increased gluconeogenesis and esterification, increases the metabolism of free fatty acids, produces ketone bodies, and eventually leads to diabetic ketoacidosis. The main clinical symptoms are ketone bodies, which can cause coma, death, and chronic hyperglycemia. Chronic high blood sugar can induce a variety of large and small vascular diabetic complications, including cardiovascular disease, kidney disease, diabetic retinitis and peripheral neuropathy. Drug discovery is imminent At present, insulin replacement therapy is the first-line treatment for the life-saving of diabetic patients. Traditionally, the focus of the pharmaceutical industry has focused on the development of innovative insulin that is more convenient and administered through other routes. It is understood that there are approximately 1.84 million people with type I diabetes diagnosed and treated in the seven major pharmaceutical markets in the world, and the market for therapeutic drugs for type I diabetes has reached $2 billion. Although insulin replacement therapy can save the lives of patients with type 1 diabetes, it does not cure the disease and can even cause hypoglycemia, but it does not prevent complications. Therefore, the development of drugs that can change the course of the disease is imminent. The immune drug market is limited Currently, the most advanced treatment strategy is to delay the progression of type I diabetes by inducing immune tolerance or regulating autoimmunity, the second immune and inflammatory response. New vaccines are under development, including the 65 kDa isoform of glutamic acid decarboxylase (GAD65) and BHT-3021. Non-antigen-specific immunomodulators, such as the CD3 monoclonal antibodies Otelixizumab and Teplizumab are also under development. Due to the mechanism of action, these drugs may harm normal immune system function. In addition, many FDA-approved immunomodulators are in clinical trials. Although the vaccine can delay or prevent the further decline of β cell function, once the patient is diagnosed with type I diabetes, only 10% to 20% of the β cells function normally. Therefore, the vaccine-based tolerability pathway is most effective for patients at high risk of developing type 1 diabetes. However, this type of treatment is less effective in patients who have already lost most of the beta cells that have lost their function. Early in the onset of Type I diabetes, non-antigen-specific immunomodulators are most effective in patients before the onset of clinical symptoms to diabetes. Overall, there is a limited market size for this type of immunomedic for treatment of diabetes, based on the limited safety of non-antigen-specific therapies or the benefit from this treatment. The regeneration route faces challenges When the metabolic needs such as pregnancy or obesity increase, the beta cell population expands. This means that it can be replicated and differentiated into beta cells through different endocrine or non-endocrine cells, thus providing hope for those who have already lost β cells to eventually recover through endogenous cells. It has been found that certain specific peptides and growth factors such as gastrin and glucagon-like peptide growth factor 1 (GLP1) can expand the β cell population and thus make animal models of type 1 diabetes lacking due to immunosuppressive agents. Blood sugar returned to normal. Two drugs approved by the FDA, dipeptidyl peptidase 4 (DPP4) inhibitors and proton pump inhibitors, increase the levels of GLP1 and gastrin in the circulation of non-obese diabetic (NOD) mice. In the human body to provide a theoretical basis for testing. Cell pathways include hepatocytes, progenitors, dendritic cells, and xenotransplants, and these pathways are currently bringing scientifically exciting opportunities. In addition, in addition to the scientific and technical challenges faced by cell-based therapeutics, they also face greater barriers to approval. Before any of these products are on the market, these issues need to be resolved. Combination drugs have potential Any regenerative treatment may be hampered by an uncontrolled autoimmune response. Therefore, the combination of safe, antigen-specific resistance therapies and upcoming regenerative therapies will be the next step in the development of new drugs. After pregnancy, the maternal immune system is slightly inhibited, thereby tolerating the development of the fetus and stimulating the expression of active factors for fetal growth and development. A recent study showed that patients with chronic type I diabetes exhibit pregnancy-induced beta-cell function and improved blood glucose. This study provided support for the use of tolerant drugs and regenerative therapies in combination with natural analogs. This combination has great potential for the development of type I diabetes modulator drugs. Market evolution is promising Judging from the current R&D channels for the treatment of type I diabetes, insulin replacement therapy will continue to dominate the market for the next 10 years. In the long run, tolerant drugs, antigen-specific and β-cell-specific regenerative drugs that are currently in early development will provide a very promising platform for the development of disease-modifying drugs. Although only one of these drugs will be marketed first, combination therapy will be the most effective way to treat type I diabetes for a long time. Related>>> Sales of new hypoglycemic agent Victoza exceeded expectations In the first quarter of this year, Victoza (liraglutide) created approximately $66 million in sales worldwide for Novo Nordisk, far exceeding company and analyst expectations. In mid-2009, Victoza successfully entered some of the major European markets. It was approved in the United States at the end of January this year and was quickly launched into the market in mid-February. At present, the drug has been successfully listed in 15 countries. Victoza is an injection that is injected once daily. 10% market share in the market for 10 weeks On April 27th, Novo Nordisk CEO Lars Rebien Sorensen said in an analyst conference call that Victoza is a GLP-1 analogue that quickly occupied 10% of the GLP-1 market 10 weeks after its listing in the United States. Under the strong push of Victoza, Novo Nordisk’s sales in the first quarter of this year reached DKK 13.7 billion, which is 9% higher than the same period last year. For this reason, Novo Nordisk has increased its sales growth forecast from 6% to 10% in the past to 7% to 10% in local currency. Victoza's sales performance has always been very exciting. The drug was approved for type 2 diabetes patients with unsatisfactory hypoglycemic effect after metformin monotherapy. The FDA requires Novo Nordisk to indicate “risk assessment and avoidance strategies†on product labels, and also put forward some mandatory post-marketing requirements. According to the FDA's requirements, given the preclinical rodent studies that have reported thyroid C-cell neoplasms, a corresponding “black-box warning†is also printed on the Victoza label. Although Victoza is entering an ever-expanding and fiercely competitive market, there is only one type of GLP-1 product that has been approved except Victoza: Amylin and Eli Lilly Launched GLP-1 Byetta (exenatide, exenatide) ), 2 injections per day. A new formulation, Bydureon, jointly developed by Amylin, Alkermes and Eli Lilly, is injected once a week and is currently undergoing FDA approval, and is expected to enter the market this year. Victoza better than Januvia Victoza's R&D program involves many direct comparison tests for other hypoglycemic agents. At the annual meeting of the American Diabetes Association in 2009, Novo Nordisk announced positive results from the LEAD-6 trial of Victoza versus Byetta. Recently, Novo Nordisk announced the results of a randomized open trial comparing the best-selling product DPP-4 inhibitor, Januvia (Sitagtin), produced by Victoza and Merck in patients with metformin-failure diabetes. , sitagliptin). Although it is generally believed that the efficacy of Januvia is not strong, this oral drug has achieved great success. In 2009, it sold 1.9 billion US dollars. The Lancet also published the results of the trial online on the same day. In this 26-week trial, hemoglobin A1c levels, fasting glucose, and body weight decreased significantly in the Victoza group compared with the Januvia group. It may be even more important that patients in the Victoza and Januvia groups have similar satisfaction with treatment or that the Victoza group is better than the Januvia group. High patient satisfaction Satisfaction of diabetes treatment questionnaire survey results showed that the improvement in overall treatment satisfaction was significantly greater in the Victoza 1.8 mg group than in the Januvia group, and the Victoza 1.2 mg group was similar to the Januvia group. There was no significant difference in the patient's perception of convenience (oral and injection). Lead investigator Richard Pratley said the results showed that injection did not affect patients' satisfaction with the treatment, and that "the injection needle is small, painless, and simple to use." In addition, as Victoza's weight loss is more pronounced, this may increase overall patient satisfaction with Victoza.
Knee Warmer is using batteries as power supply, to reduce pains of the knees. It provides stable heat penetrating so as to relax fatigue caused to the knees.
It is always with velcro for fastening better, which also enhance the heat therapy effect, as it touches better to human knees.
Below picture for reference, it can be made in different shapes and designs.
Knee Warmer Knee Warmers for Arthritis, Specialized Knee Warmers, Electric Knee Warmer, Thermal Knee Warmer Ningbo JustLive Electrical Appliance Co., Ltd , https://www.makeheat.net