Lab Equipment,Medical Equipment,Ball Mill,Blood Bank Instrument Guangdong Widinlsa International Co.Ltd , https://www.gdwidinlsa.com


Determination of 7 Polybrominated Diphenyl Ethers (PBDEs) in Aquatic Products by Gas Chromatography Triple Quadrupole Mass Spectrometry (TSQ8000)
Li Chunli
Thermo Fisher Scientific (China) Co., Ltd.
Summary
In this paper, an analytical method for the determination of polybrominated diphenyl ethers in aquatic products using a triple quadrupole GC/MS (GC-MS/MS) was developed. Soxhlet extraction was carried out after adding the internal standard BDE-77 to the sample. The extract was degreased by an automatic GPC system, purified by a multi-layer silica gel column, and separated on a 15 m long capillary gas chromatography column using GC-MS. /MS's Multiple Reaction Monitoring Mode (Timed-SRM), characterized by retention time and ion pair (parent and daughter ion) information, quantifies ion pairs with high response values. The results showed that the detection limit of the method was 0.1 pg/ul, and the relative standard deviation was 2.29-5.58%.
Foreword
Polybrominated diphenyl ethers (PBDEs) are brominated flame retardants (BFRs), which are widely used in building materials, textiles, chemicals, and electronics due to their excellent flame retardant efficiency and thermal stability. Electrical and other industries. The demand for PBDEs has increased dramatically in the world in the past 10 years. Since the early 1980s, decabromodiphenyl ether has become the largest bromine-containing flame retardant in China.
As an added flame retardant, PBDEs are easily released into the environment from products, especially during electronic waste stacking and recycling. In 1981, the presence of PBDEs was found in barracudas, crickets and sea otters in Sweden [ 1], then PBDEs were detected in marine fish, mussels, and sediments. [2] In 1987, Jansson et al. [3] first proposed to classify PBDEs as a class of global environmental pollutants. PBDEs are detected from air, water and human body, and their content has increased rapidly in the world in recent years [4~8]. A detailed survey of POPs in Swedish breast milk shows that other POPs in breast milk are more Chlorinated biphenyls showed a decreasing trend from the 1970s to the 1990s, but the PBDEs content has been increasing [9]. Since then, Europe, North America, Japan, China and other regions have carried out research on PBDEs pollution in humans.
In this study, an automated GPC combined with multi-layer silica gel column purification technology for sample pretreatment-gas chromatography triple quadrupole mass spectrometry was established to determine the polybrominated diphenyl ethers in aquatic products. This article describes the use of Thermo Fisher Scientific's new generation triple quadrupole gas chromatography mass spectrometer (TSQ8000) for the analysis of seven PBDEs in aquatic products. The secondary mass spectrometry scan greatly reduces the influence of background interference in complex matrix samples and improves the detection sensitivity of the target compound. The method has the advantages of low sensitivity, good stability and wide linear range.
Experimental part
Instruments and reagents
Mass Spectrometer: TSQ8000 Mass Spectrometer (Thermo Fisher Scientific, USA);
Gas Chromatograph: Trace 1310 GC with AI 1310 Autosampler (Thermo Fisher Scientific, USA);
Column: TG-5MS 15m*0.25mm*0.1μm capillary column;
Reagents: dichloromethane, ethyl acetate, cyclohexane, n-hexane: pesticide residue level;
Instrument method
Gas phase method:
Column oven: 100 ° C (maintained 1.5 min), 35 ° C / min to 200 ° C, 25 ° C / min to 280 ° C (maintained for 5 min); inlet: splitless injection, no split time: 1 min, liner: Inert non-split (Cat. No. 453A1925), inlet temperature is 280 ° C; carrier gas: constant current, 1.5 ml / min; transmission line: 280 ° C
Mass spectrometry method :
The ion source temperature is 300 °C , using the Acquisition-Timed method, SRM scanning, and the specific detection ion pair is shown in Table 1:
Table 1. Mass spectrometry conditions for seven polybrominated diphenyl ethers and internal standards
Name
RT
Window
Mass
Product Mass
Collision Energy
BDE28
5.29
0.6
405.8
246
10
BDE28
5.29
0.6
407.8
248
15
BDE47
6.04
0.6
326.8
247.9
10
BDE47
6.04
0.6
485.7
325.7
15
BDE47
6.04
0.6
487.7
325.9
15
BDE77-ISTD
6.35
0.6
486
326
15
BDE77-ISTD
6.35
0.6
486
377
25
BDE100
6.6
0.6
403.8
296.9
30
BDE100
6.6
0.6
563.6
403.8
20
BDE99
6.79
0.6
405.8
296.9
25
BDE99
6.79
0.6
565.6
405.8
20
BDE154
7.24
0.6
485.7
376.8
30
BDE154
7.24
0.6
643.8
485.7
10
BDE153
7.5
0.6
485.7
376.8
30
BDE153
7.5
0.6
643.8
485.7
10
BDE183
8.14
0.6
485.7
376.8
30
BDE183
8.14
0.6
561.6
454.7
35
BDE183
8.14
0.6
721.4
561.6
10
Pretreatment method
Extraction: Accurately weigh 5.0g of fish sample (accurate to 0.01g) into the cellulose extraction sleeve, add 1 ng of internal standard BDE-77 standard solution, and add appropriate amount of anhydrous sodium sulfate to Cover with glass wool and perform Soxhlet extraction with 200 mL of n-hexane/acetone (1:1, v/v) solution for more than 12 h (about 4 reflows/h). After Soxhlet extraction, the extract was steamed to dryness and the fat content was calculated gravimetrically. The residue was dissolved in ethyl acetate/cyclohexane (1:1, v/v) solution to a volume of 6 mL and purified by GPC.
GPC Purification: Transfer the above extract to an automated gel purification system using a low pressure packed column with a column size of 2 cm × 50 cm, a packing of 50 g Bio-Beads S-X3, a sample tube to a volume of 6 mL, and a 5 mL injection . The mobile phase was ethyl acetate / cyclohexane (1:1, v / v) solution, flow rate 5 mL / min,
Isocratic elution. The UV detection wavelength was 240 nm. The effluent component of 20-40 min was collected by an automatic solvent evaporation system and evaporated to near dryness under reduced pressure, and re-dissolved by adding 2 mL of n-hexane to be purified in the next step.
Multi-layer silica gel column purification: fill a 1.5cm × 10cm glass column with appropriate amount of glass wool, and then fill 1 cm high anhydrous sodium sulfate, 1g neutral silica gel, 3g acidified silica gel, 1g neutral silica gel from bottom to top. And 1 cm high anhydrous sodium sulfate. Pre-rinse with 10 mL of n-hexane, transfer the GPC purification solution to the column, elute with 20 mL of n-hexane, and elute with 20 mL of n-hexane/dichloromethane (1:1, v/v) solution. All eluates were collected and rotary evaporated for at least a volume, transferred to a vial, blown dry with nitrogen, reconstituted with 100 μL of n-hexane, and transferred to an end cannula for injection.
Analysis of results
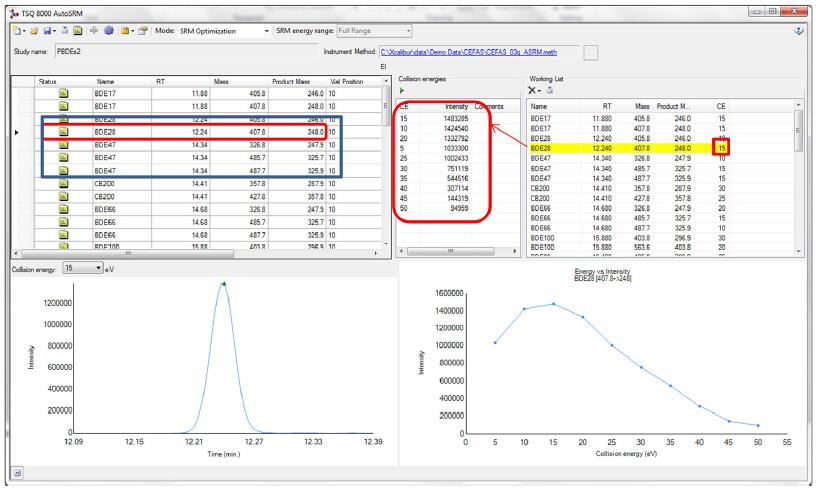
Target secondary mass spectrometry optimization - AUTO SRM effect display: AUTO SRM only needs to prepare the target mixture, the whole process is automated, no complicated instrument method editing and data browsing, the optimization results are intuitive, specific ion pairs of specific compounds, according to The collision energy is optimized, and the system automatically sorts the response of the ion pair. We only need to select the channel with the highest response. See the picture below, taking BDE28 as an example.
A needle injection performed a gradient of 5-50 on the CE collision energy, and automatically aligns the ion response intensity. The system has selected the highest response energy, which is the optimal secondary mass spectrum information. At the same time, more than 40 kinds of targets can be optimized in one experiment, and the obtained ion pair information can be directly imported into the instrument method editing interface for data acquisition, which greatly improves the work efficiency.
Chromatographic separation results
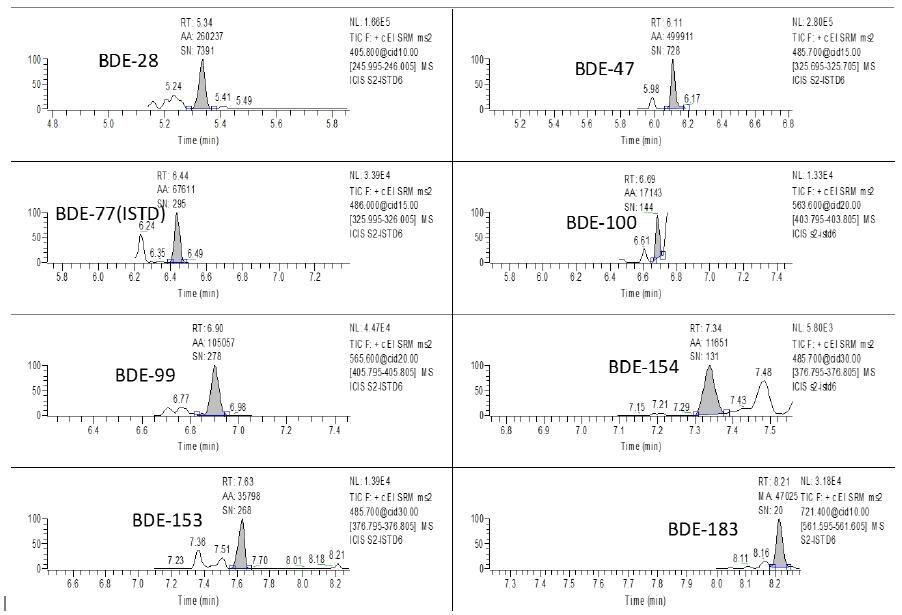
The SRM mass spectrometry conditions (parent ion-subion-collision energy) of each compound can be determined by optimizing the collision energy by Auto-SRM mode. See Table 1 for the SRM chromatogram of 7 polybrominated diphenyl ethers. .
Standard curve and minimum limit of quantitation
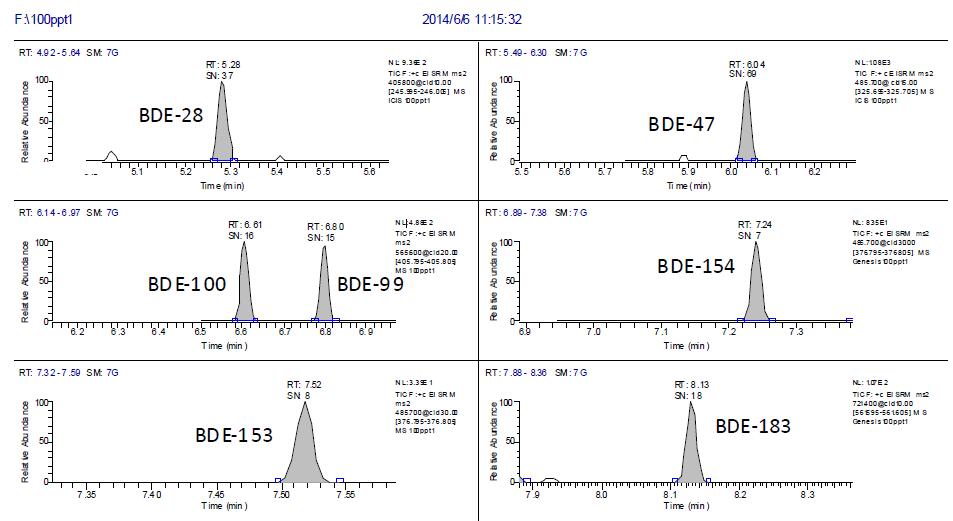
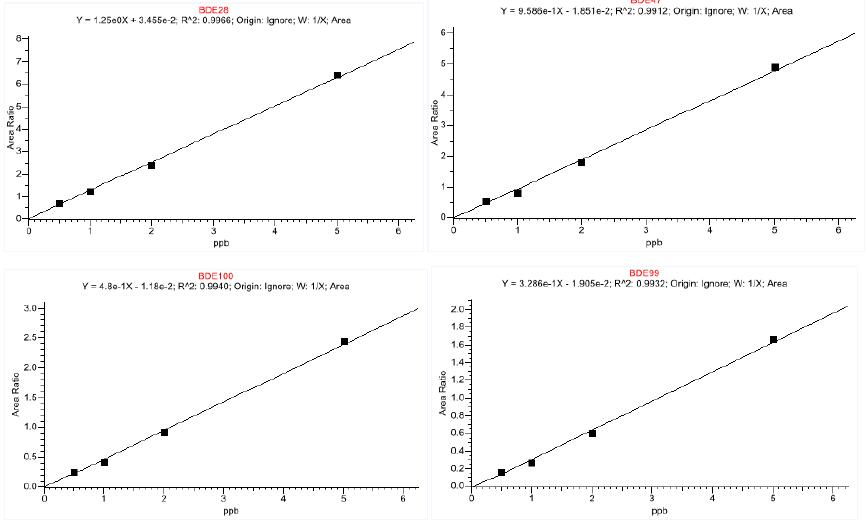
According to EU standards, standard materials and internal standards (PBDE-77, 10 pg/ul) were added to the negative samples, and the concentration of PBDEs was 0.5pg/ul, 1pg/ul, 2pg/ul, 5pg/ul, according to 2.2. Perform chromatography-mass spectrometry. With S/N=3 as the detection limit, the detection limit spectrum is shown in Figure 3. It can be seen from Figure 3 that the S/N= of the seven PBDEs is greater than 3. Taking the standard solution concentration as the abscissa (X), the chromatographic peak area of ​​the quantitative ion pair is plotted on the ordinate (Y), and the regression equation is obtained. The linearity of the 7 PCBs in the range of 0.5pg/ul to 5pg/ul is good, the correlation coefficient is greater than 0.991, and the linear equation is shown in Fig. 4. The detection limit of this method is better than the reported value in the literature, which can ensure the qualitative and quantitative detection of 7 PBDEs residues in the sample.
Figure 3. Chromatogram of 0.1 pg/ul of 7 polybrominated diphenyl ethers
Figure 4. Linear relationship diagram of seven polybrominated diphenyl ethers
Actual sample detection and method precision determination
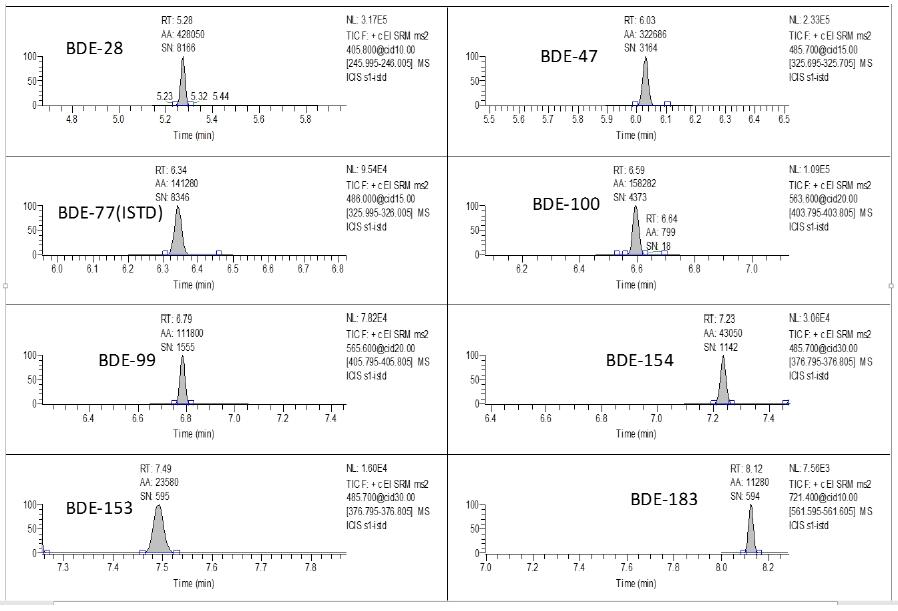
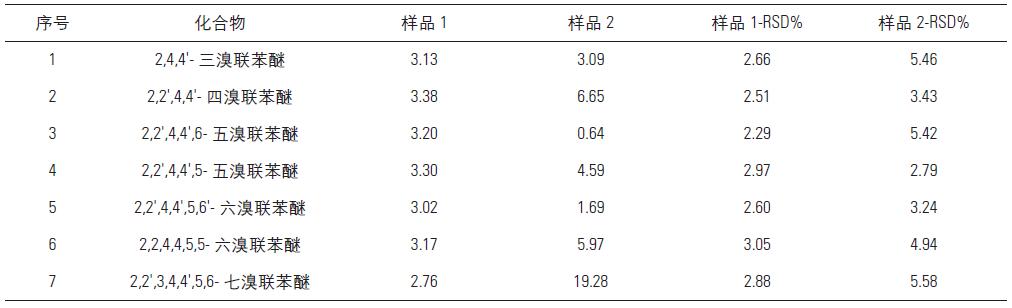
According to the experimental method, two actual samples were analyzed and tested, and 7 samples were continuously injected for each sample, and the relative standard deviation RSD% of each compound was calculated. Experimental Results Table 2 shows that the target organic pollutants are contained to varying degrees in both samples. The relative standard deviation (RSD, n=7) of the test method is 2.29-5.58%.
Figure 5. Chromatogram of each target detected in sample 1.
Figure 6. Chromatogram of each target detected in sample 2.
Table 2. Precision of sample test results and methods
in conclusion
The method uses ThermoFisher's new generation triple quadrupole mass spectrometer TSQ8000 to determine the residues of seven polybrominated diphenyl ethers in aquatic products, with high sample extraction rate and convenient operation. The instrument has the advantages of good selectivity, wide linear range and high sensitivity. At the same time, the ion pair scanning provided by the TSQ8000 can greatly eliminate the interference of false positives, thus making the detection result more accurate. In a complex matrix, the relative standard deviation (RSD, n=7) of the instrument is 2.29-5.58%. The minimum detection limit for the seven PBDEs involved in this analytical test method was 0.1 pg/ul. It can fully meet the testing requirements of PBDEs in the EU and countries.
references
1. Andersson O, Blomkvist G. Polybrominated aromatic pollutants found in fish in Sweden. Chemosphere, 1981, 10: 1051-1060.
2. Watanabe I, Kashimoto T, Tatsukawa R. Polybrominated biphenylethers in marine fish, shellfish and river and marine sediments in Japan. Chemosphere, 1987, 16: 2389-2396.
3. Jansson B, Asplund L, Olsson M. Brominated flame rerardantsubiquitous environmental pollutants? Chemosphere, 1987, 16:2343-2349.
4. Li A, Rockne KJ, Sturchio N, et al. Polybrominated diphenylethers in the sediments of the Great Lakes. 4. Influencing factors, trends, andimplications. Environ Sci Thehnol, 2006, 40:7528-7534.
5. Rayne S, Ikonomou MG, Antcliffe B. Rapidly increasing polybrominated diphenyl ether concentrations in the Columbia River System from 1992 to 2000. Environ Sci Technol, 2003, 37:2847-2854.
6. Song W, Ford JC, Li A, et al. Polybrominated diphenyl ethers in the sediments of the Great Lakes 3 Lakes Ontario and Erie. Environ Sci Technol, 2005, 39: 5600-5605.
7. Kim BH, Ikonomou MG, Lee SJ, et al. Concentrations of polybrominated diphenyl ethers, polychlorinated dibenzo-pdioxins and dibenzofurans, and polychlorinated biphenyls in human blood samples from Korea. Sci Total Environ, 2005, 336:45-56.
8. Thomsen C, Lundanes E, Becher G. Brominated flame retardants in archived serum samples from Norway: A study on temporaltrends and the role of age. Environ Sci Technol, 2002, 36: 1414-1418.
9. Sudaryanto A, Kajiwara N, Takahashi S, et al. Geographical distribution and accumulation features of PBDEs in human breast milk from Indonesia. Environ Pollut, 2007, 151: 130-138.