Corrosion Inhibitor,Pbtc Salts ,Scale Inhibitor Water Treatment Chemicals and biocides Co., Ltd. , http://www.nspharmainter.com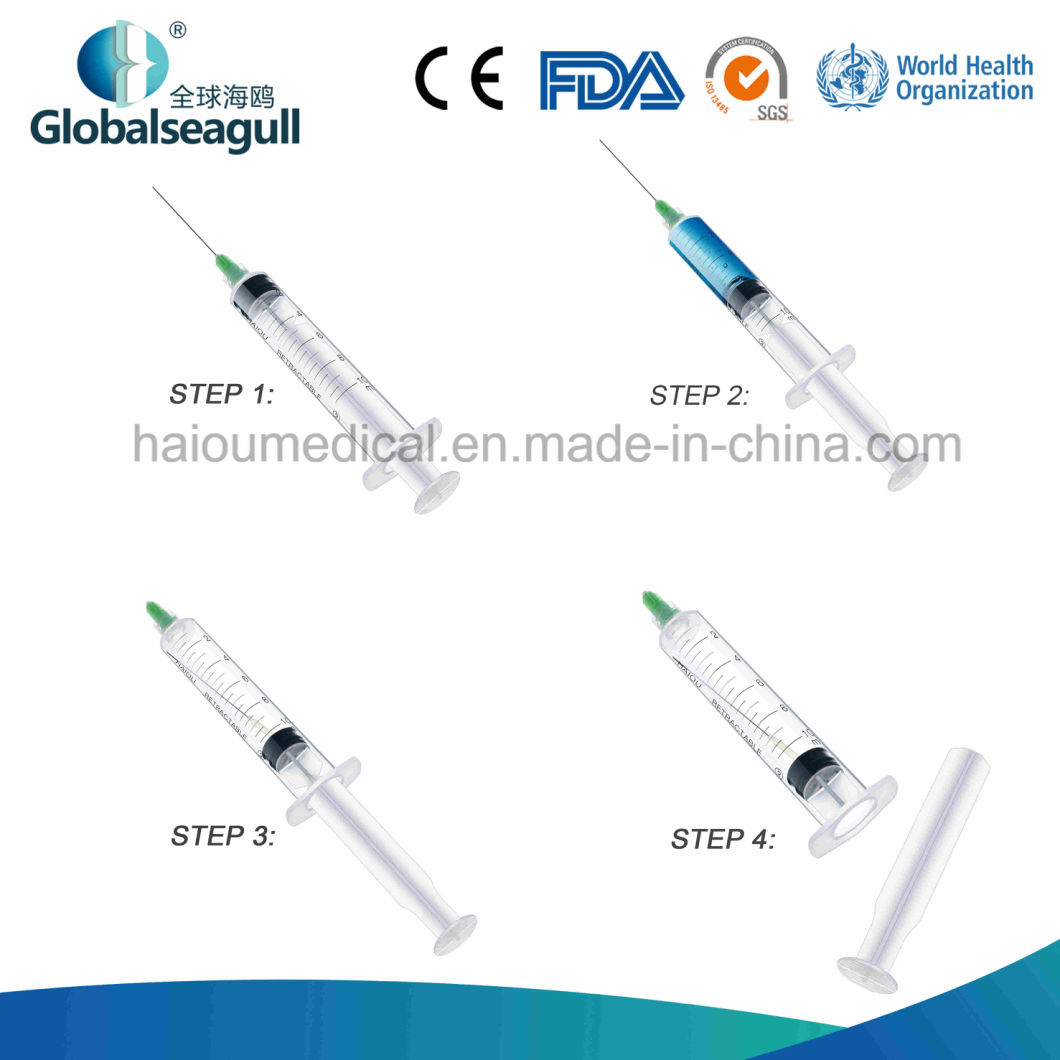
Product's detail:
Size:
0.05ml,0.5ml,1ml, 2ml, 3ml, 5ml, 10ml;
Nozzle:Â
luer lock/fixed needle
Needle size:
18G--30G, customized;
Material:
Medical grade PP;
Graduation:
Indelible ink, easy readability, accurate and clear scale marking;
Lubricant:
Silicone oil;
Sterile:
EO gas, non-toxic, non-pyrogenic;
Packing:
Blister/PE package, 100 PCS in one box, carton packing outside;
Shelf life:
3 years;
Brand:
Haiou or OEMÂ
Certificate:
CE, ISO,FDA, WHO PQSÂ
Products advantage:
1. After injection, needle will be retracted into barrel to reach completely destroy.
2. Prevent reuse, Safety, Auto-destruct, protect patients and medical practitioners.
3. Low residue and reduces medication waste, reduce hazardous waste volume, protect environment.
4. Avoid the cross infection after injection.
5. With CE, ISO, FDA, WHO PQS certificates.Â
Company Introduction:
Haiou Medical Apparatus Co., Ltd. was founded in 1997. It is a high-tech enterprise which specialized in R&D, manufacturing and marketing in sterile medical apparatus. Haiou Medical is committed to solving the infection problem in the filed of Injection in medical institutions. Replying on our own professional technology and studying, we also research and develop AD syringe, Needle retractable safety syringe and Auto retractable safety syringe and so on. Thanks hard effort of our team, we have been passed ISO13485 and CE Certificate, our AD syringe and Safety syringe also passed the WHO PQS pre-qualification, the safety syringe also passed the FDA registration in May, 2015.
Replying on years domestic and international sales experience, at present, we has been built sales market in Europe, America, Southeast Asia, Middle East, Africa and etc. And it has been won long term support from customers by high quality products, high grade after-sales service and sincere spirit of cooperation.
Certificates:
Production process:
Packing and delivery:
Our team:
Contact: Johnson Lau
MOB:+86-18948433991
TEL: +86-663-2930777Â Â
Fax: 0663-2905999Â Â Â Â
Web: www.haiou.net.cn
Needle Retractable Safety Syringe:
Product's detail:
Size:
0.05ml,0.5ml,1ml, 2ml, 3ml, 5ml, 10ml;
Nozzle:Â
luer lock/fixed needle
Needle size:
18G--30G, customized;
Material:
Medical grade PP;
Graduation:
Indelible ink, easy readability, accurate and clear scale marking;
Lubricant:
Silicone oil;
Sterile:
EO gas, non-toxic, non-pyrogenic;
Packing:
Blister/PE package, 100 PCS in one box, carton packing outside;
Shelf life:
3 years;
Brand:
Haiou or OEMÂ
Certificate:
CE, ISO,FDA, WHO PQSÂ
Products advantage:
1. After injection, needle will be retracted into barrel to reach completely destroy.
2. Prevent reuse, Safety, Auto-destruct, protect patients and medical practitioners.
3. Low residue and reduces medication waste, reduce hazardous waste volume, protect environment.
4. Avoid the cross infection after injection.
5. With CE, ISO, FDA, WHO PQS certificates.Â
Company Introduction:
Haiou Medical Apparatus Co., Ltd. was founded in 1997. It is a high-tech enterprise which specialized in R&D, manufacturing and marketing in sterile medical apparatus. Haiou Medical is committed to solving the infection problem in the filed of Injection in medical institutions. Replying on our own professional technology and studying, we also research and develop AD syringe, Needle retractable safety syringe and Auto retractable safety syringe and so on. Thanks hard effort of our team, we have been passed ISO13485 and CE Certificate, our AD syringe and Safety syringe also passed the WHO PQS pre-qualification, the safety syringe also passed the FDA registration in May, 2015.
Replying on years domestic and international sales experience, at present, we has been built sales market in Europe, America, Southeast Asia, Middle East, Africa and etc. And it has been won long term support from customers by high quality products, high grade after-sales service and sincere spirit of cooperation.
Certificates:
Production process:
Packing and delivery:
Our team:
Contact: Johnson Lau
MOB:+86-18948433991
TEL: +86-663-2930777Â Â
Fax: 0663-2905999Â Â Â Â
Web: www.haiou.net.cn
Model NO.: HO-NRSS
Group: Children
Needle: With Needle
Syringe Use: Intramuscular Injection
Disinfection: Disinfection
Medical Device Regulatory Type: Type 3
Nozzle: Luer Lock or Fixed Needle
Trademark: Haiou
Transport Package: Blister Package
Specification: 0.05ml, 0.5ml, 1ml, 3ml, 5ml, 10ml
Origin: Guangdong, China
HS Code: 90183100
Model NO.: HO-NRSS
Group: Children
Needle: With Needle
Syringe Use: Intramuscular Injection
Disinfection: Disinfection
Medical Device Regulatory Type: Type 3
Nozzle: Luer Lock or Fixed Needle
Trademark: Haiou
Transport Package: Blister Package
Specification: 0.05ml, 0.5ml, 1ml, 3ml, 5ml, 10ml
Origin: Guangdong, China
HS Code: 90183100
Needle Retractable Safety Syringe: