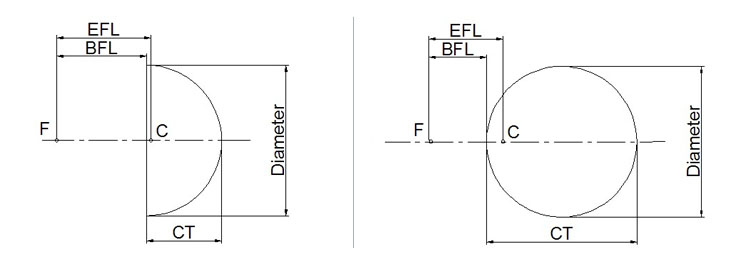
Ball Lenses are commonly used to improve signal quality in fiber coupling applications, or for use in endoscopy or bar code scanning applications. Ball Lenses feature short back focal lengths to minimize the distance needed from the Ball Lens to the optical fiber.
Worldhawk can provide spherical ball lenses and half ball lenses in a range of substrates for performance in the ultraviolet to the NIR. Such as including H-K9L(N-BK7), Sapphire, CaF2, BaF2, ZnSe or custom materials upon request.
Half Ball Lens,Optical Half Ball Lens,Optical Glass Ball Lenses,Optical Glass Half Ball Lens ChangChun Worldhawk Optics Co.,Ltd , https://www.worldhawk-optics.com While China's manufacturing has become increasingly attractive on the international pharmaceutical market, the road to internationalization of preparation products has not been so "smooth."
While China's manufacturing has become increasingly attractive on the international pharmaceutical market, the road to internationalization of preparation products has not been so "smooth."
According to statistics, in the export of pharmaceutical products in China, more than 90% of the pharmaceutical products are occupied by APIs, while the formulation products are still less than 10%, and most of the formulation exports are mainly concentrated in developing countries.
"It can be said that the internationalization of the formulation bears the dream of a generation of pharmaceutical people. Of course, we also know that it is not something that can be achieved in the short term," said Fang Daxing, deputy general manager of the international business of Sinopec Pharmaceuticals, in an interview with the reporter.
There is growth but the degree of acceptance is not high With the rise of the pharmaceutical market in developing countries, the global pharmaceutical market share in countries such as Europe, the United States, and Japan has begun to decline, and the global pharmaceutical landscape has quietly changed.
"China has been regarded as one of the most attractive, most comprehensive and most valuable emerging markets in the world by foreign companies," said Meng Dongping, vice president of the China Chamber of Commerce for the Import and Export of Chinese Medicines and Health Products.
Especially in the past 10 years, the average growth rate of pharmaceutical products in China has exceeded 20%. Last year's total value of imports and exports reached 73.3 billion U.S. dollars, an increase of 39%, which is almost twice the national growth rate of imports and exports, once again set a new record.
"It can be said that from the traditional advantage industries of Western medicine raw materials to proprietary Chinese medicines, from medical excipients to diagnostic and therapeutic equipment and the emergence of new products and new technologies, China's pharmaceutical and health products have never been welcomed by the international market like today." Ping said.
Taking formulation products as an example, the total export volume in 2002 was only 200 million U.S. dollars, and in less than 10 years, it increased to 2.2 billion U.S. dollars. And domestic pharmaceutical companies can produce almost all pharmaceutical dosage forms, and the export market has gradually become diversified.
Up to now, there are more than 30 pharmaceutical manufacturers in China that have passed the quality certification of the European Union, the United States, Japan, and the World Health Organization (WHO). "This has laid a solid foundation for China's preparations to develop international competition and open the international market." Meng Dongping said that last year, domestic pharmaceutical companies exported pharmaceutical products to 174 countries and regions.
However, in fact, occupying half of the country's exports is still the Asian market. The market in Europe and the United States is still dominated by APIs, accounting for more than 50% of total pharmaceutical exports, and the proportion of exports of pharmaceutical preparations is less than 4%, and it is mainly foreign-funded enterprises.
Internationalization can not be "one size fits all"
So, in the end what restricts domestic formulation products from entering the international market?
Fang Daxing believes that first of all, the European and American regulatory system is more complicated. Usually, the preparation enterprises in China in the United States and the European Union have to complete a product registration and it takes almost 3 to 4 years or even longer. Because products are relatively single, it is difficult to enter foreign wholesale and retail systems and medical insurance systems, and it is difficult to achieve large-scale sales.
Secondly, the overall level of domestic drug quality standards setting is low, especially in the production of the preparations, registration procedures, including the submission of documents are very different, which has become an obstacle to international registration and certification.
In addition, there are several thousand companies engaged in the export of pharmaceutical preparations in China, and the phenomenon of low-level repetition is more serious, resulting in a decline in quality and prices. In particular, the frequent emergence of counterfeit drugs in recent years has cast a shadow on the international image of Chinese medicine.
Although there are various unfavorable factors, going abroad is still a favorite choice for many companies.
“Because not only can we obtain more market opportunities, but we can also absorb more management experience in the international market through full competition and experience in the international market to feed back companies and gain competitiveness in the domestic market,†said Meng Dongping.
But Niu Lili, director of research and development at Shenzhen Lijian Pharmaceuticals, believes that this may not be suitable for all Chinese pharmaceutical companies.
“Although the standard of China's new GMP has been very close to European standards, the specific implementation process is still not the same.†Niu Lili said that even if the company is determined, supported by people, financially supported, and supported by relevant guidance parties, The most important must also continue to ensure the quality level.
And this is not a certificate that can be solved.
From this point of view, it seems that preparation products entering the international mainstream market are not only problems of certification, but also involve marketing channels, bidding, insurance, and later market management.
"From the perspective of current market development, R&D of R&D drugs has already withdrawn from the mainstream of the market. The fight against generic drugs is time, speed, low-cost product development, and market preemptive injection." Meng Dongping believes that in order to adapt to the development trend of globalization Domestically, we should establish an international marketing network that suits the characteristics of Chinese products as soon as possible, make more contributions in the construction of drug supply chains, and make more efforts to provide strong policy support.
The pharmaceutical companies themselves also need to make necessary adjustments, grasp as much as possible and as quickly as possible the frontier information, reduce costs, and strengthen channel construction.
“Enterprises must have long-term planning, a clear overall view, and lay out according to the laws of the market. They must also have a solid capital investment. Investment is not just about equipment. The key is to have good management concepts and outstanding teams. In order to advance planning, Fang Xingxing said that domestic pharmaceutical companies usually prefer to use price advantage to seize foreign markets, but they must focus on key issues and treat them differently.
For example, Tianjin Tasly and Tong Ren Tang, which mainly produce proprietary Chinese medicines, have developed their own characteristics in the construction of international marketing networks. “They usually select one or two market targets as the mainstay, and after forming advantages, they will gradually radiate to neighboring countries and regions.â€
However, Meng Dongping reminded that domestic pharmaceutical companies must not ignore the follow-up to the traditional market when they are developing new markets. "We must learn to walk on two legs. Otherwise, there may be a situation where we lose "watermelon and sesame seeds."
Half-Ball (hemispherical) lenses are ideal for applications such as fiber communication,endoscopy, microscopy, optical pick-up devices,and laser measurement systems.