1.Good safety of gelatin-free.The first lyophilized Varicella Vaccine, containing no gelatin from animals, invented and produced in China. Getting rid of gelatin from varicella vaccine can significantly decrease the ratio of anaphylaxis incidence .
2.Long validity period by good stability. The first approved varicella vaccine with 36 months of validity period in the world. Adopting BH-2 stabilizer with own IP rights (Chinese patent granted No.: ZL200910138411.6, International patent application No.: PCT/CN2009/001405) greatly enhances the stability of the product and ensures the validity period for 36 months under the storage condition.
3.Better protection with high titer and immune eff Varicella Vaccine(Live) Live Bioproducts Pharmaceutical,Live High Potency Medicine,Live Preventive Pharmaceutical,Varicella Biopharmaceutical Live Changchun BCHT Biotechnology Co.,Ltd , https://www.ccbcht.net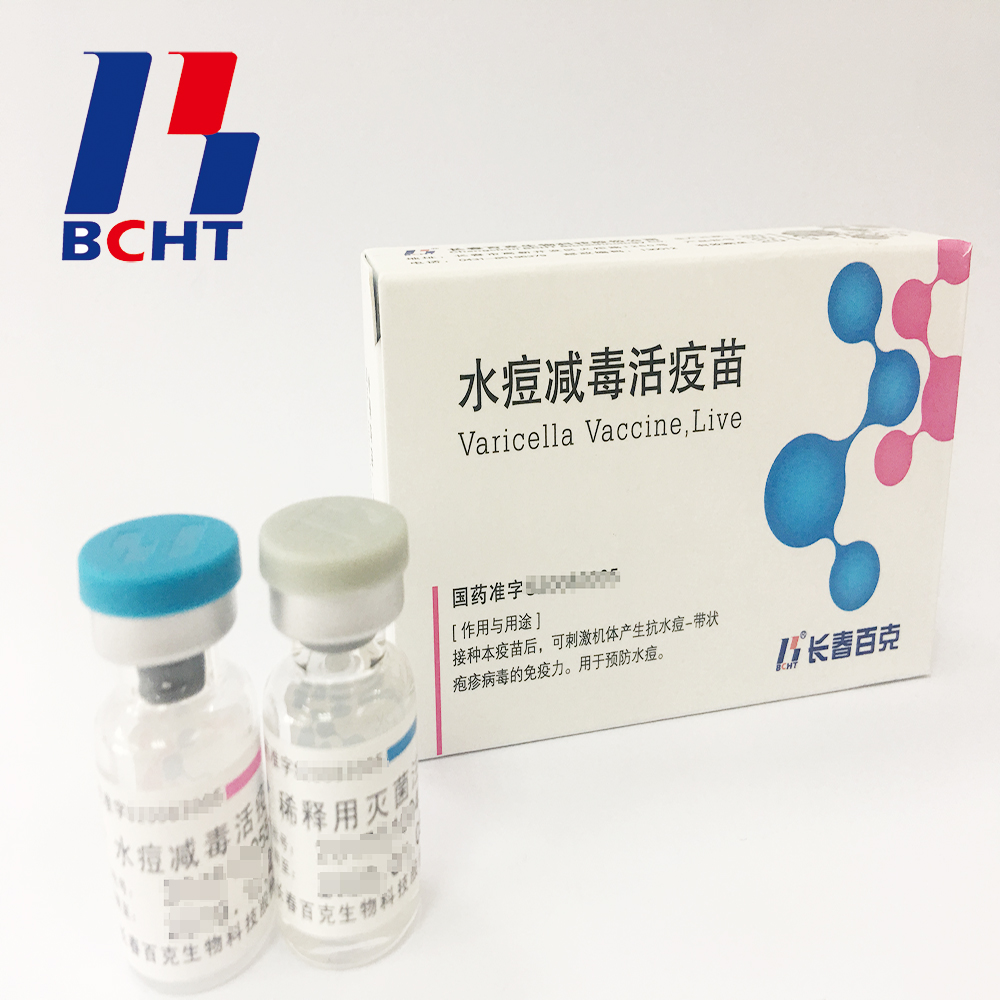 icacy. It`s documented that compared with the varicella vaccine of low titer, high titer vaccine can reduce breakthrough cases by nearly 75% in vaccination. Of all released batches of Varicella Vaccine from BCHT, the titers are not less than 10000 PFU/dose tested by National Institutes for Food and Drug Control.
icacy. It`s documented that compared with the varicella vaccine of low titer, high titer vaccine can reduce breakthrough cases by nearly 75% in vaccination. Of all released batches of Varicella Vaccine from BCHT, the titers are not less than 10000 PFU/dose tested by National Institutes for Food and Drug Control.
[Humidity] Keep the indoor relative humidity at 60%-65%; the relative humidity of the incubator is 55%-60%; the relative humidity of the hatcher is 65%-70%. ã€Temperature】Incubation room and incubation room temperature is kept at about 25°C; egg surface temperature is maintained at about 39°C in the early stage of incubation; 37.5-38°C is maintained at the late stage of incubation; the hatcher temperature is generally controlled at 36-37°C. should. [turning the egg] In order to keep the eggs uniformly heated and maintain the normal embryonic development, turn the eggs on time. When the fire pit hatches, the eggs can be turned once every 4 hours. The machine is hatched and turned every 2 hours. The angle of turning eggs is 90 degrees. ã€Cool egg】12-13 days after hatching, cool eggs twice a day should be regularly so that the heat generated by the fetus inside the eggs can be distributed in time to prevent "natural" death. The temperature of cool egg should be controlled at about 36°C, that is, when it is connected with human skin, it can be warm and not cool. [Ventilation] While maintaining normal temperature and humidity, it is necessary to pay attention to frequent ventilation, so that the air in the room or incubator is always kept fresh.